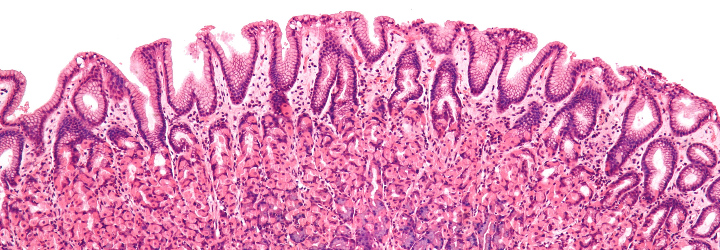
To expound upon my previous post regarding histology and pathology, there is an important step between conducting a biopsy and generating a diagnosis. The tissue in question must be “fixed” (preserved), sectioned (cut into slices), and stained [Origin: Old Norse steina (to paint)]. There are numerous stains and different ones are useful for different purposes. The classic stain that most students of histology are introduced to is the H&E stain. This stands for haematoxylin and eosin.
Haematoxylin
Origin: Greek, haima (blood) + xylos (wood)
Extracted from the logwood tree; depending on its preparation, its solution appears blood red
Eosin
Origin: Greek, eos (dawn)
So named after the Greek goddess of the dawn, Eos, for its red/dawny color
To start, haematoxylin is oxidized into haematein and combined with aluminum ions. This forms the active dye-metal complex, and this complex is what binds to nucleic acids to stain nuclei and ribosomes purple. (The former contains DNA in the form of chromatin and the latter is composed of RNA.) It is thought that the highly negative phosphate backbone is what binds to the positively-charged haematein. We use the word basophilic [Gk. base + philia (love)] to refer to entities that bind to basic, i.e., positively-charged dyes. I will discuss the etymology of chemical terms such as “base” in a future post!
Eosin is used as the counterstain to haematoxylin. When eosin is dissolved in water or ethanol, its carboxylic acid and phenol are deprotonated to form a negative ion. This form of eosin binds to positively-charged entities such as the positive amino acids, e.g., arginine and lysine. Hence, the cytoplasm appears red because its proteins bind to the negatively-charged eosin. We use the word eosinophilic [Gk. eosin + philia (love)] to refer to entities that bind to acidic, i.e., negatively-charged dyes.
In the 1970s, the price of haematoxylin rose steeply, and it resulted in a search for a synthetic alternative. My schoolmate and former histology TA, Dr. Alexander Morgan, relayed an interesting aside to me: “European lust for haematoxylin based dye led to British logging in South America, and why there’s one tiny, mosquito-infested, swampy country in South America where they speak English [referring to Belize]. Many people died (dyed?) of malaria trying to harvest the wood to make the stain.”
These terms present a natural tie-in to immunology. There are two leukocytes that I am referring to: one is the eosinophil, which is colored quite intensely red, and the other is the basophil, which is colored quite intensely blue. I believe a picture is in order to better illustrate:
Another interesting stain is the silver stain. Camillo Golgi, a 19th-century Italian physician and Nobel laureate, developed silver staining to study the nervous system. (The cellular organelles known as Golgi bodies are named after him.) There are two classes of cells that take up silver: the argentaffins and the argyrophiles.
Argentaffin
Origin: Latin, argentum (silver) + affinis (akin/affinity)
Affinity for silver; more specifically, cells that take up silver without a reducing agent
Argyrophile
Origin: Greek, argyros (silver) + philia (love)
Love of silver; more specifically, cells that take up silver and need a reducing agent
There are various methods for preparing different silver stains. These stains can be used for a number of applications such as:
- Diagnostic microbiology (staining of fungi such as Pneumocystis jiroveci)
- Neuropathology (staining of neuritic plaques and neurofibrillary tangles in Alzheimer disease)
- Histology (staining of type III collagen and reticulin)
Left: neurofibrillary tangles of Alzheimer disease. Right: membranous nephropathy
The last stain I will briefly touch upon is the periodic acid-Schiff (PAS) stain. This stains for polysaccharides, glycoproteins, glycolipids, and the like. Hence, it can be used to diagnose things like the glycogen storage diseases, adenocarcinomas (which often secrete mucin), Whipple’s disease, etc. The reason why I bring this up is that many people say it incorrectly. It is not periodic as in “recurring time intervals.” Rather, is per-iodic, i.e., of the periodiate ion (IO4–)! Periodic acid oxidizes vicinal [L., vicinus (near)] diols to create a pair of aldehydes. These aldehydes then react with the Schiff reagent to produce a purple color.