When we think of the deadliest diseases of the world, things like Ebola and the Black Death come to our minds. However, it turns out that cardiovascular disease (CVD) is the world’s leading cause of death in this day and age. In the 1970s, we were convinced that CVD was caused by dietary fat. Indeed, there was a public campaign to stamp out fat and replace it with carbohydrates. People didn’t realize that fat wasn’t the issue. Rather, fat has the highest calorie/mass ratio (9 calories per gram) and excess consumption of fat leads to obesity and its milieu of problems, including CVD. The food industry replaced fat with carbohydrates to meet this craze, and “fat-free” foods were instead pumped with sugar. Of course, calories are calories—and when people consumed these foods in earnest, not much changed with regards to the incidence of CVD.
So what exactly is fat? Chemically, fat is a glycerol molecule + three fatty acids. Fatty acids are long-chained aliphatic carboxylic acids that are either saturated (only single bonds) or unsaturated (one or more double/triple bonds). Quick aside: in popular culture, we hear about “omega-3 fatty acids” and “omega-6 fatty acids.” This refers to the position of the first double bond in the fatty acid, with the omega position being the last carbon in the chain (see picture below). So an omega-3 fatty acid is a fatty acid that has its first double bond on the 3rd carbon from the omega position.
Glycerol
Origin: Greek, glykeros (sweet) + –ol (chemical suffix for alcohols)
So named because glycerol is, in fact, sweet (and is an alcohol)
Aliphatic
Origin: Greek, aleiphar (oil)
Refers to hydrocarbons that do not contain benzene rings
What about the term lipid [Gk. lipos (fat)]? In the vernacular, people equate the word “lipid” to “fat,” and its etymology certainly vindicates such an equation. But of course, we must be rigorous with our definitions. To start, fats (i.e., triglycerides) are certainly a lipid subclass. Other subclasses include waxes (fatty acid esters such as beeswax; article’s featured image) and sterols (isoprene-derived steroids such as cholesterol). One classical definition of a lipid is the following: lipids are hydrophobic (some also include amphiphilic) small molecules that can dissolve in organic solvents but not water.
Hydrophobic
Origin: Greek, hydor (water) + phobos (fear)
Fear of water, i.e., not dissolvable in water or other polar solvents
Amphiphilic
Origin: Greek, amphi – (both) + philia (love)
Love of both water and fat, i.e., dissolvable in polar and nonpolar solvents
For those who love math, recall that for a statement to be proven true, it must be true in all instances. For a statement to be proven false, one need only provide a counterexample. So my counterexample for this definition of lipid is the molecule carbon tetrachloride (CCl4). CCl4 is a small molecule, it is hydrophobic, it dissolves in organic solvents, and it doesn’t dissolve in water. Now, to patch the definition up, one can make an addendum to include “naturally-occurring.” Needless to say, CCl4 is not naturally-occurring. Is there another definition of lipid that you prefer? Let me know in the comments!
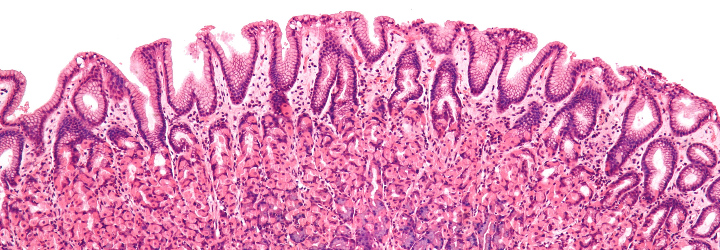
Before ending this post, I would like to touch on the significance of fat in disease. There are a number of disease states that manifest with malabsorption; one of them is coeliac disease. The diarrhea that coeliac patients experience after ingesting gluten is more accurately termed steatorrhea:
Steatorrhea
Origin: Greek, stear (fat) + rhein (to flow)
Flow of fat (through the intestines), i.e., fat in the stools
How can you identify steatorrhea? If the stool is especially foul-smelling and sticks to the sides of the porcelain shrine, you’ve got yourself a flow of fat.